Medical Devices
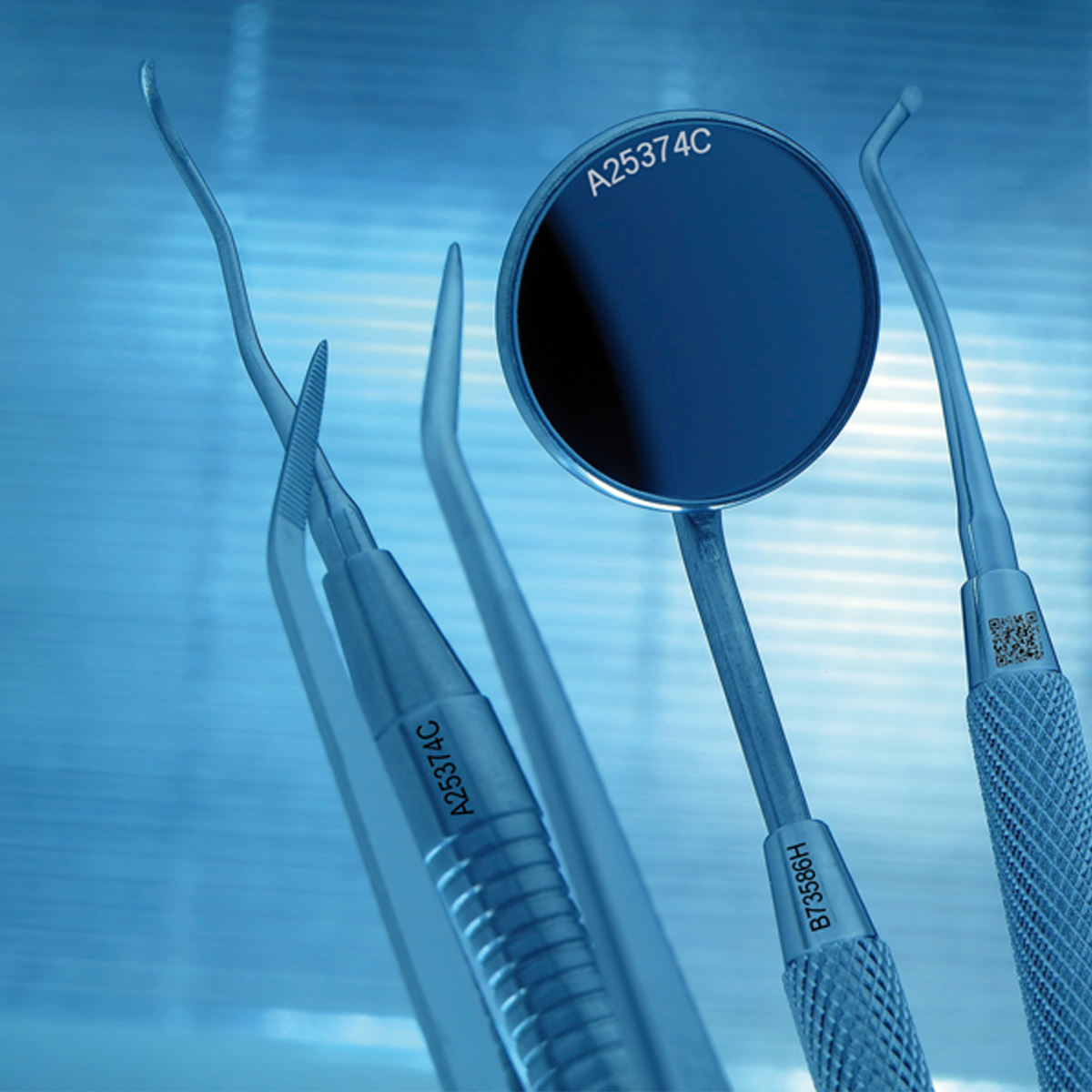
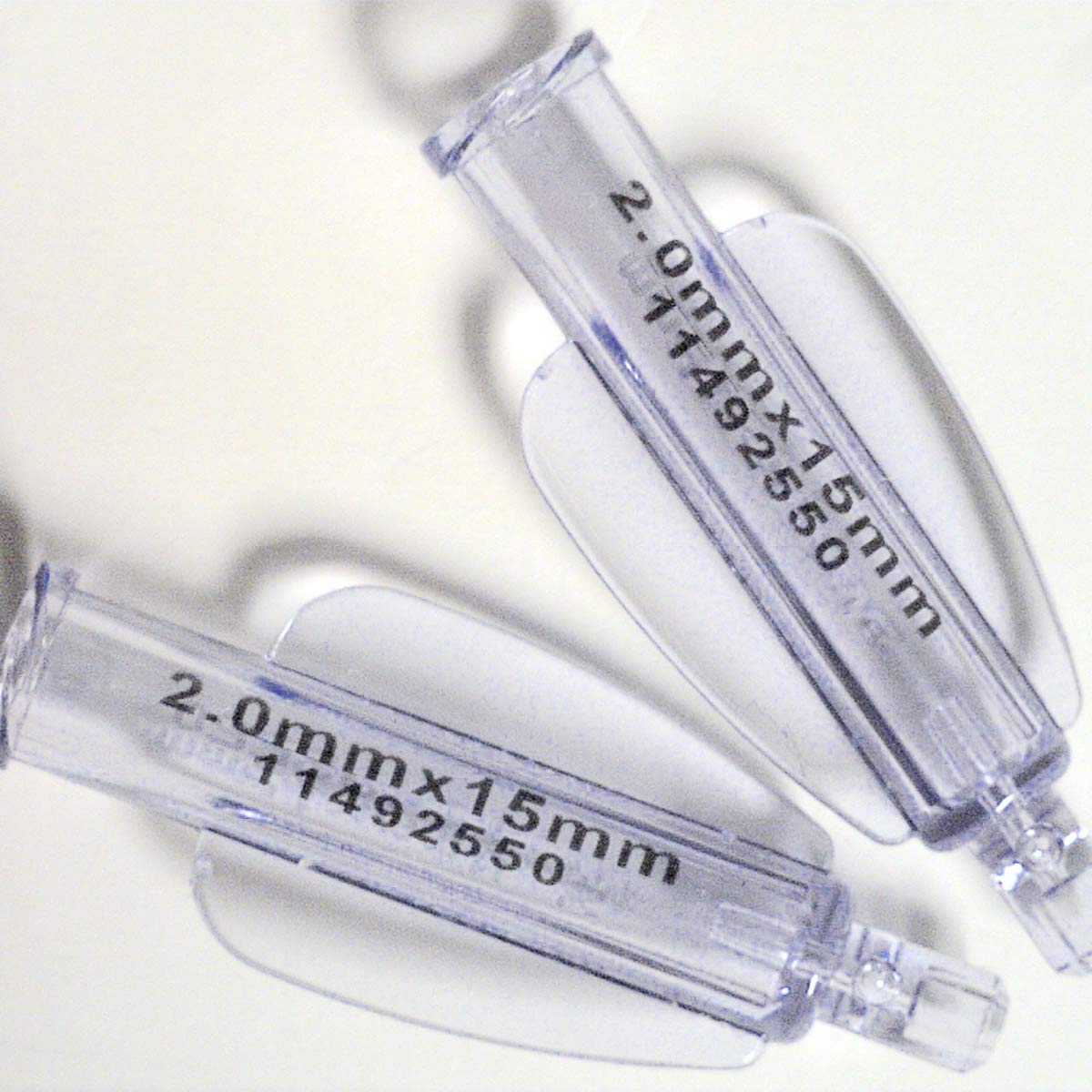
All medical devices and tools need to be permanently marked to facilitate identification and traceability.
Manufacturers of components for medical devices mark their products to identify them, to add instructions and to enable tracking and traceability. Industry standards spearheaded by organisations like GS1, are being implemented across the industry. Lasers are able to produce surface marks with no depth that do not harbour bacteria and pose a hygiene risk.Materials
Plastics – PC, PVC, PMMA, Nylon 66
Metals – Stainless steel, titanium
Products
Surgical instruments
Dental equipment
Pacemakers
Clamps
Syringes
Test Kits